软骨发育不全
软骨发育不全 is a rare genetic bone growth condition characterized by disproportionate short stature, 脊柱弯曲和头部增大(大头畸形).
Our thoughtful approach to clinical trial design enables us to move quickly and efficiently while prioritizing the safety of participants.
Clinical trials evaluate the safety and 功效 of investigational medicines and are required to support regulatory approval of new medications. 在药物被批准后, clinical trials also play an important role in helping further understand the effects of treatment. An observational study is another type of clinical research through which researchers collect data and assess health outcomes without changing participants’ care.
Learn more about the clinical trials 博彩平台网址大全 is conducting below. If you are interested in learning more about a particular trial, please email medinfo@bmrn.com.
软骨发育不全 is a rare genetic bone growth condition characterized by disproportionate short stature, 脊柱弯曲和头部增大(大头畸形).
CLN2 (late infantile neuronal ceroid lipofuscinosis type 2) disease is an ultra-rare and rapidly progressing pediatric brain disorder and one of the most common forms of neuronal ceroid lipofuscinosis, a group of inherited disorders collectively known as Batten disease.
杜氏肌营养不良(DMD)是一种隐性疾病, X-linked neuromuscular disorder caused by a severe deficiency or the complete absence of dystrophin, 一种保护肌肉细胞的蛋白质.
A型血友病 is a rare bleeding disorder caused by a mutation in the gene that provides instructions to make the protein FVIII, 什么是血液正常凝结所必需的.
HAE is a rare genetic disorder characterized by spontaneous swelling that can be life-threatening by blocking airways and preventing breathing. The main type of HAE is caused by mutations in the SERPING1 gene. This gene encodes a protein called C1-INH which plays an important role in controlling certain types of swelling in the body. When C1-INH is missing or not working correctly, sudden, unexpected swelling events can occur.
Hypochondroplasia is a rare genetic condition generally characterized by short stature, 矮壮的构建, 胳膊和腿短得不成比例, 广泛的, 手脚短, 轻度关节松弛, 脊柱侧凸, 和巨头.
议员IVA, 也被称为Morquio A, is a rare inherited disease that affects major organ systems in the body. The condition is a form of mucopolysaccharidosis, which is a type of lysosomal storage disorder.
粘多糖病(议员六世), 也被称为马罗托-拉米综合征, is an inherited lysosomal storage disorder caused by the deficiency of an enzyme normally required for the breakdown of certain complex carbohydrates.
Phenylketonuria (北大) is an inherited metabolic disorder caused by a deficiency of the enzyme phenylalanine hydroxylase (PAH).
A clinical trial is a research study done to evaluate new potential treatments in people. 在临床试验期间, information is collected to determine whether a product candidate is safe and effective, 以及评估药物的风险和益处. 有关临床试验的更多信息,请访问 临床试验.政府 or The Center for Information and Study on Clinical 研究 Participation
研究 and development occurs in several stages before clinical trials commence in people. Preclinical testing is conducted for each investigational product to evaluate the safety, 功效, 管理的最佳实践, and many other properties before entering human clinical trials. 一旦临床前测试完成, an Investigational New Drug (IND) application is submitted to a regulatory authority, 比如美国.S. 美国食品药品监督管理局, so the regulator can evaluate the safety of the product candidate and ensure that clinical trial participants will not be subjected to an unreasonable risk.
临床试验分四个阶段进行:
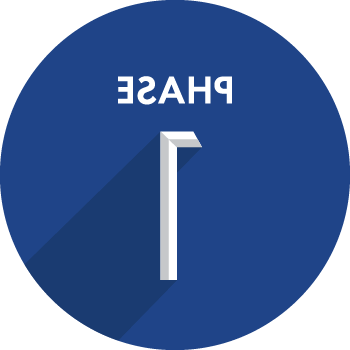
研究用药物在人体中进行评估, 通常是在没有这种情况的志愿者身上, for the first time to evaluate its safety and best practices for administering the product candidate.

评估候选产品 to determine a safe dose or range of doses, 进一步评价其安全性, and to begin testing in participants with the condition of interest to determine if it has the intended or predicted effects.

评估候选产品, 通常在持续时间较长的大型试验中, 确认其有效性并进一步评价其安全性. Phase 3 trials often compare the study drug to commonly used treatments (if any) or to placebo treatment, 如果这样做在科学上和伦理上都是合适的.

Post-marketing studies are performed after regulatory agency approval occurs. These studies are designed to collect additional information including the medicine’s risks, 在更广泛的患者群体中的益处和最佳使用, 通常在很长一段时间内. 例如, a Phase 4 study could be a registry of patients with a certain condition to collect medical information and better understand outcomes, 无论是接受药物治疗的患者还是未接受药物治疗的患者.
1-3期临床试验完成后, 向监管机构提交监管申请. The application contains all data gathered about the safety and effectiveness of the study drug from the preclinical studies and clinical trials that have been performed. 该应用程序还包含有关化学的信息, 毒理学, 产品的药理学和制造工艺. 监管机构审查数据,然后, 如果得到批准, the new medicine can be marketed and distributed to the public through prescription by a qualified physician.
在临床试验中测试潜在的新疗法时, it is critical to include the 广泛的est possible range of individuals, to ensure these medicines have the best chance of improving outcomes for the diversity of patients who will ultimately receive them. 在博彩平台网址大全, we create pioneering medicines for people with genetic conditions around the world, and we are deeply committed to enrolling representative populations in our clinical trials.